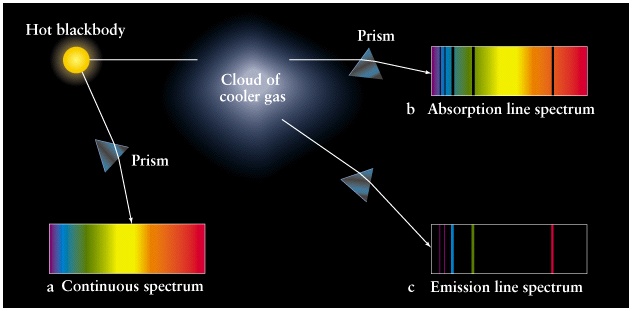
Each line in the spectra represents the energy


Emission Spectrum of Hydrogen
Lecture 6: Discrete Spectra of Atoms

The bright-line spectra produced by four elements are represented in the diagram below. Given the bright-line spectrum of a mixture formed from two of these

Climate Prediction Center - Monitoring and Data: Global Temperature Monitoring
SOLVED: The bright-line spectra for three elements and a mixture
SOLVED: May someone please help me! Thank you. 25) The bright-line spectra produced by four elements are represented in the diagram below: Bright-Line Spectra of Four Elements Wavelength (nm) 750 700 650
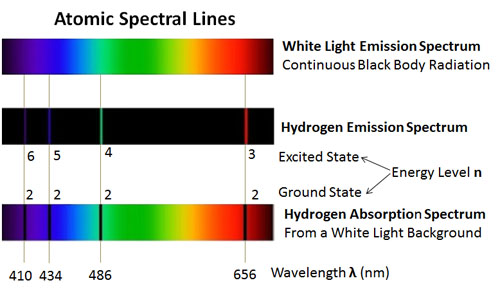
Atomic Spectral Lines

unit 4 periodic table and electron scan text and graphics 2019 2020 review.docx - Chemistry Name Mr. Krueger Period Unit 4 Periodic Table

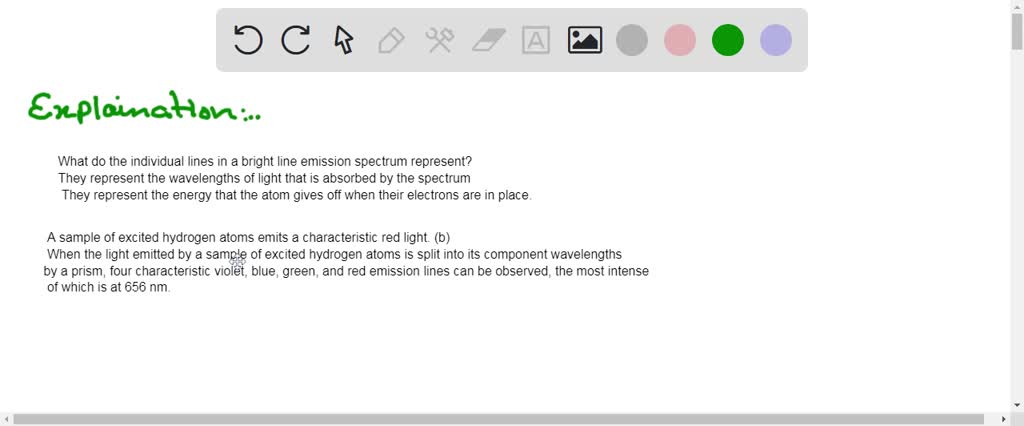
What do the lines in an emission spectrum represent? - Quora
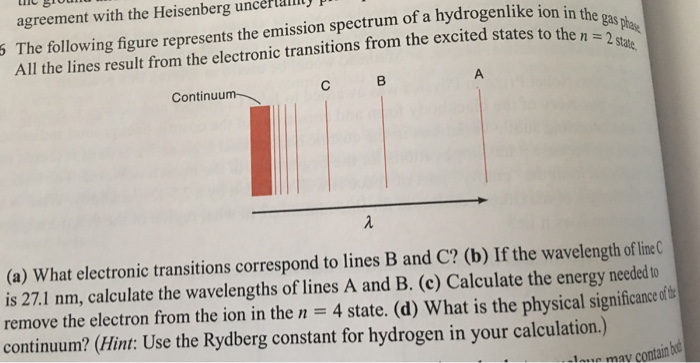
Solved The following figure represents the emission spectrum

Kami Export - GENESIS PEREZ GIL - Emission Spectra WS.pdf - 1. 2. The characteristic bright-line spectrum of an element is produced when

Chemistry Final Study Guide 낱말 카드

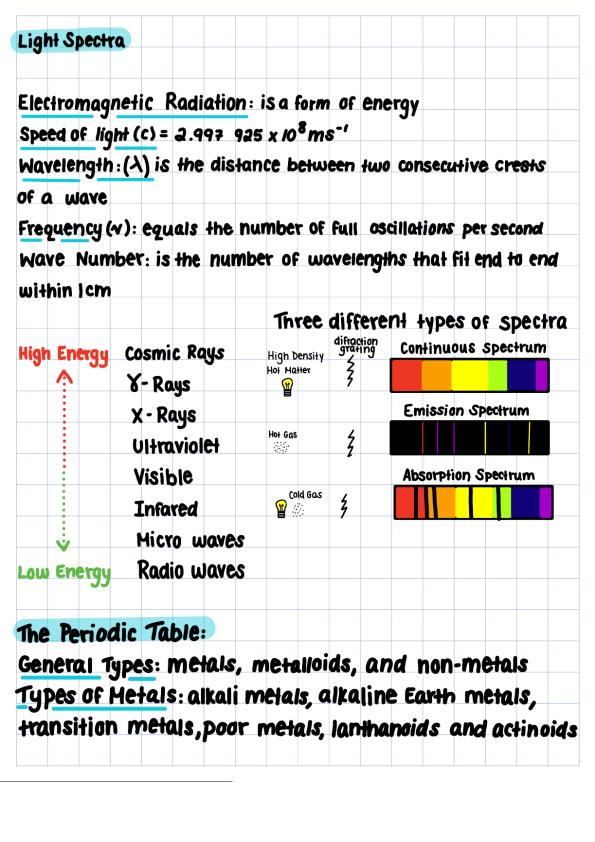
Spectra lines - Definition, Classification, Types, broadening